
Recently FDA cleared medical inventions
From labs to labels: ASU’s cutting-edge medical devices win global recognition and FDA approval
ASU is a university on the forefront of biomedical ingenuity, even without a traditional in-house medical school. ASU-linked medical inventions and patents routinely garner international renown, major donor funding and – eventually – FDA approval for clinical application.
Along with its wealth of creative and dedicated students and faculty, ASU owes the success of these biomedical enterprises partly to numerous research partnerships with renowned institutions across the nation, coupled with a diverse array of interdisciplinary research institutions like the Biodesign Institute, which uses natural ecological systems to create sustainable solutions that address healthcare’s most complex global challenges.

Cloud-based computational modeling
platform illustrates surgical outcomes
Take EndoVantage, whose revolutionary software platform performs hypothetical “surgeries” on patients considering high-risk procedures. Using 3-D modeling technology that generates images based on a patient’s CT scan, EndoVantage’s Surgical Preview software simulates endovascular implantations and subsequent blood flow changes without penetrating a single cell in a patient’s body. Physicians can then project likely surgical outcomes across hundreds of individualized anatomical profiles, allowing them to anticipate the success of procedures that were once very expensive shots in the dark. With this software, EndoVantage hopes to help medical engineering companies create more effective, affordable and life-saving devices.


The FDA recognized EndoVantage’s disruptive potential late last year, following the company’s four-year engagement with Skysong Innovations. Their venture began in 2013, when Skysong partnered with the Mayo Clinic to sponsor a grant competition promoting personalized, patient-centered medicine. Led by inventors and researchers from both ASU and Mayo, the EndoVantage team took home the $100,000 top prize. Meanwhile, EndoVantage found a new home at Skysong Innovations’ research and business center.
Backed by Skysong’s $20,000 in seed funding, coaching team and commercialization resources, EndoVantage nabbed a further $250,000 from the Arizona Commerce Authority the following year.

A brighter outlook for jaundice
with NeoLight
ASU student Vivek Kopparthi is responsible for another life-saving venture that Skysong helped take from prototype to startup to licensing—leading all the way to FDA clearance. When Kopparthi and his research team in the business, engineering, biotechnology and industrial design schools developed a portable LED incubator with the potential to cure jaundice, Skysong immediately took note. Later, the FDA followed suit, granting Kopparthi’s NeoLight and SkyLife phototherapy systems 501(k) clearance for marketing at home and abroad.

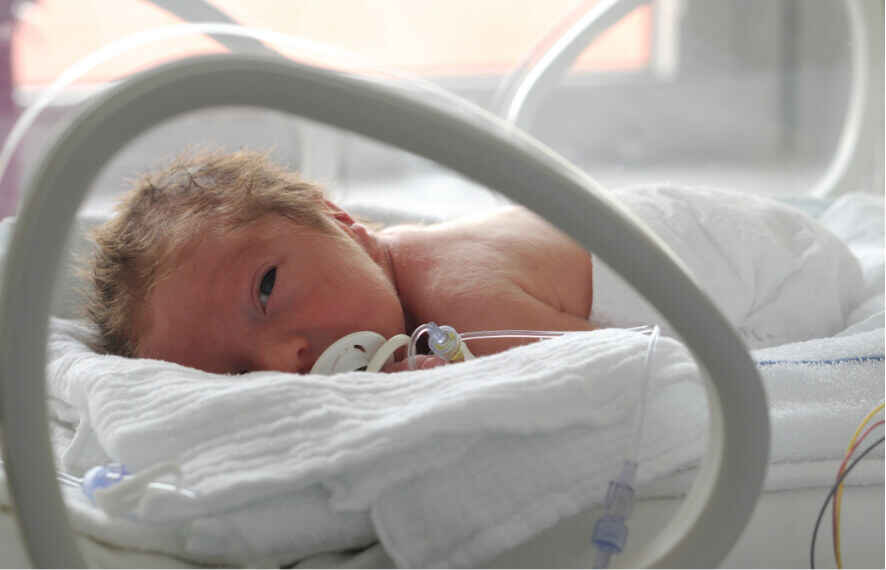
Jaundice, which affects an estimated 60% of newborns each year, can be deadly if left untreated, which it often is in underdeveloped and developing areas like parts of Africa and India, Kopparthi’s home. Most incubators are expensive even by U.S. standards and cause disturbing side effects in already ill newborns. In poorer nations, treatment can be prohibitively costly, cumbersome and environmentally unsustainable. As a result, up to 12,000 infants die each day from untreated jaundice.